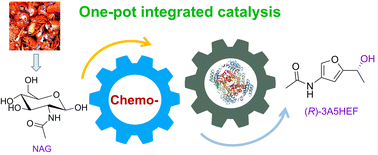
Engineering carbonyl reductase for one-pot chemobiocatalytic enantioselective synthesis of a value-added N-containing chiral alcohol from N-acetyl-d-glucosamine
[ad_1]
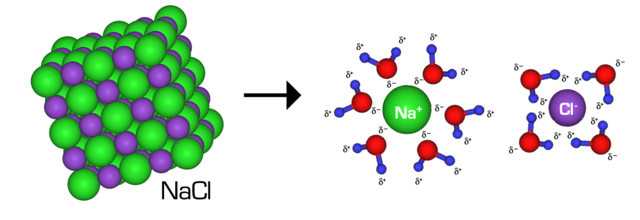
Direct chemobiocatalytic conversion of biomass into value-added chemical compounds is promising but difficult, due to the mixed benefits of the 2 applied sciences and their incompatible points. On this work, structure-guided engineering of carbonyl reductase from Streptomyces coelicolor (ScCR) was carried out to enhance its catalytic exercise and stability, thus permitting for direct conversion of chitin-derived N-acetyl-D-glucosamine (NAG) into chiral 3-acetamido-5-(1-hydroxyethyl)furan (3A5HEF) by integrating its variants with chemical catalysts, with no isolation of intermediates. Upon three rounds of site-directed mutagenesis, two sturdy variants M3 (S167F/P168S) and M4 (S167Y/P168S) have been recognized, with 6-fold greater catalytic effectivity (okaycat/Okaym) towards 3-acetamido-5-acetylfuran (3A5AF) than the father or mother enzyme and improved stability. Mechanistic insights into the improved actions of variant M3 have been offered on the idea of the molecular docking research and molecular dynamics simulations. Chemobiocatalytic conversion of NAG into (R)-3A5HEF was carried out by way of sequential chemical dehydration by tyrosine hydrochloride/CaCl2 and biocatalytic uneven discount by variant M4, with 53% yield and >99% ee. Within the scale-up two-step synthesis, (R)-3A5HEF was obtained with 42% remoted yield. Along with 3A5AF, enormously improved catalytic actions of the 2 variants towards different carbonyl compounds, significantly acetophenones, have been noticed (as much as 41-fold greater exercise than the father or mother enzyme). Numerous carbonyl compounds have been decreased to the goal chiral alcohols by the variants, with 46–99% yields and >99% ee. This work demonstrates that protein engineering is a robust technique to deal with the incompatibility between chemo- and biocatalysts in chemobiocatalysis.
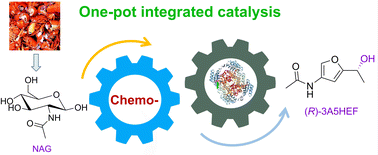
[ad_2]