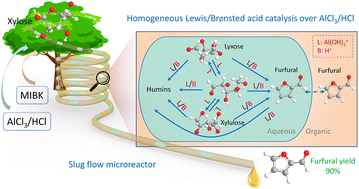
Insights into the response community and kinetics of xylose conversion over mixed Lewis/Brønsted acid catalysts in a circulate microreactor
[ad_1]
The catalytic impact of Lewis acid on xylose conversion to furfural has been extensively reported, whereas the underlying response community and kinetics should not absolutely elucidated. This work presents experimental and kinetic modelling research on xylose conversion to furfural, utilizing AlCl3/HCl as mixed Lewis/Brønsted acid catalysts in monophasic water or a biphasic water–methyl isobutyl ketone system in a circulate microreactor. The response community and kinetics have been developed, the place Al(OH)2+ was recognized because the catalytically lively species for isomerization between xylose, lyxose and xylulose, whereas each Al(OH)2+ and H+ catalyze the sugar dehydration to furfural and aspect reactions resulting in humins (e.g., sugar condensation and furfural degradation). The marketing impact of AlCl3 is attributed to not solely the tandem catalysis by way of xylulose, but in addition the parallel Al(OH)2+-catalyzed sugar dehydration. Inside the studied vary (120 to 180 °C, 0.05–0.4 M HCl, 0.04–0.12 M AlCl3), the furfural selectivity relied primarily on the focus ratio of HCl to AlCl3 moderately than their particular person concentrations or the temperature. The volcano-like evolution of the utmost furfural yield with rising HCl/AlCl3 ratio is said to (i) the decrease response orders in Al(OH)2+ for reactions forming furfural than for aspect reactions; and (ii) a gradual shift of the dominant response pathway from the Al(OH)2+-catalyzed one in the direction of H+-catalyzed one. An optimized furfural yield as much as 90% was achieved with 40 mM AlCl3 and 100 mM HCl at 160 °C from 1 M xylose in a steady slug circulate microreactor. The catalytic aqueous part could possibly be recycled and reused 4 occasions with out efficiency loss.
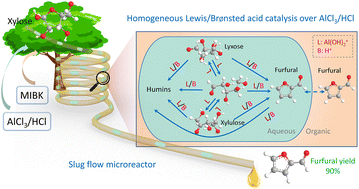
[ad_2]