Intramolecular Forces | ChemTalk
[ad_1]
Core Ideas
An intramolecular power is a power that holds atoms collectively to kind a molecule. There are three foremost varieties of these forces: covalent, ionic, and metallic, and every leads to totally different chemical properties. Intramolecular forces shouldn’t be confused with intermolecular forces, that are forces brought on by interactions between separate entire molecules.
Subjects lined in different articles
What are Intramolecular Forces?
Intramolecular forces are the forces that maintain atoms collectively to kind molecules. There are a number of totally different ways in which atoms can work together with one another, which leads to several types of forces between them. There are three foremost varieties, that are covalent, ionic, and metallic. Every power will maintain the molecule collectively in several methods, giving substances with numerous corresponding properties.
Intramolecular vs. Intermolecular Forces
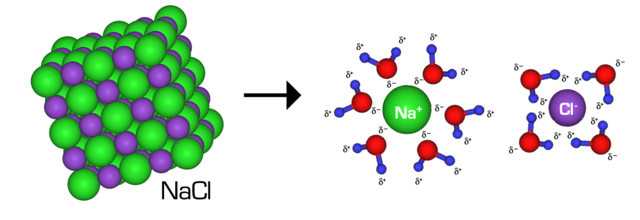
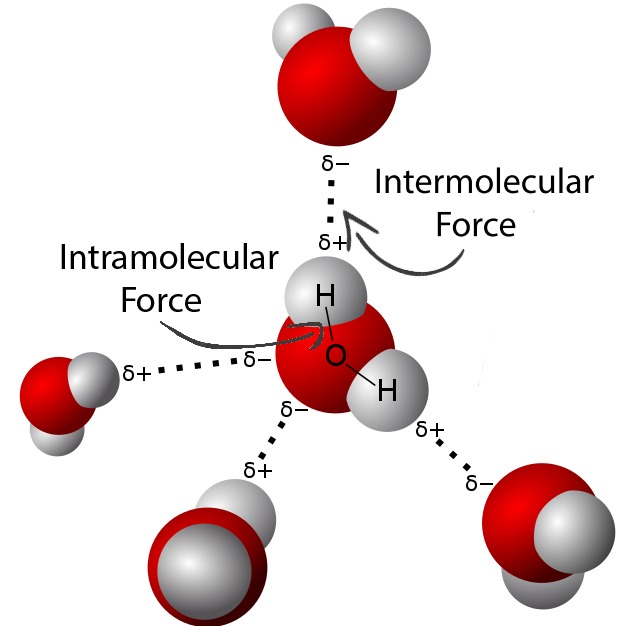
Regardless of being an analogous sounding phrase, intramolecular forces are totally different than intermolecular forces. Whereas intramolecular forces are between atoms inside a molecule, intermolecular forces are interactions between separate entire molecules, such because the hydrogen bonding that happens when a number of water molecules are close to one another. The various varieties of intermolecular forces additionally create various properties in substances, however the kind of intermolecular power between molecules is closely influenced by the cost or polarity of a molecule, which comes from its intramolecular power. In distinction to the above instance, an intramolecular power in a water molecule can be the bond that retains the 2 H atoms hooked up to the O, and it’s due to the polarity of the covalent bond that hydrogen bonding happens the way in which it does, as proven under. For a extra in-depth analogy about how these forces are associated, see right here.

Kinds of Intramolecular Forces
As beforehand acknowledged, there are a number of varieties of intramolecular forces. Every kind creates several types of molecules, and subsequently totally different properties of the substances.
Covalent bonds
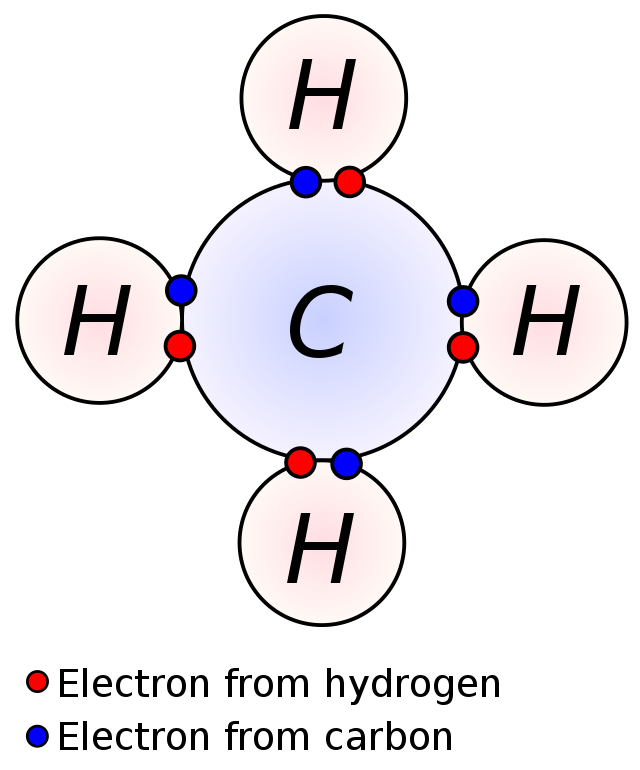
Covalent bonds are the kind of power by which two atoms share an electron pair. These happen between two atoms which are looking for an octet (be taught extra about octets and the octet rule right here!), the place the atoms will concurrently maintain the identical electron pair of their respective valence shells, bonding them to one another. The diagram exhibits how two atoms share an electron pair, leading to a bond. This may occur to create both a polar bond or a nonpolar bond, relying on every atom’s electronegativity (affinity to tug electrons near them, study electronegativity in additional element right here).
Polar Covalent Bonds
When a covalent bond happens between two atoms with totally different electronegativities, the bond is polar. It’s because one of many atoms may have a stronger affinity for the electrons than the opposite, and can pull them nearer to itself than the opposite atom. This creates a dipole, giving one atom a partial damaging cost whereas the opposite has a partial constructive cost. In some circumstances, dipoles could be so robust that they work together with surrounding ions or different polar molecules. These can be examples of intermolecular forces, which have been mentioned earlier.

Nonpolar Covalent Bonds
Conversely, covalent bonds between atoms of the identical electronegativity produce nonpolar covalent bonds. A bond is nonpolar if neither atom pulls on the electrons stronger than the opposite, and so they find yourself completely shared between them. Oftentimes, this implies two of the identical atom covalently bonding to at least one one other the place the electronegativities are precisely equal, nonetheless there are additionally nonpolar covalent bonds between totally different atoms with extremely related electronegativities, similar to between carbon and hydrogen.
Ionic Bonds
Not like covalent bonds, ionic bonds don’t share electrons. As an alternative, an ionic bond happens when one atom donates an electron to a different one close by. This occurs when a steel and a nonmetal each search an octet. The steel will fully switch considered one of its valence electrons to the close by nonmetal, producing oppositely charged ions. These now secure ions then entice one another due to the opposing expenses, creating an ionic power between them.

Metallic Bonds
Metallic bonds are a bit totally different than the opposite two varieties. They solely happen inside metals, and since metals are inclined to have a free maintain on their electrons, the forces between steel atoms are distinctive. The nuclei of steel atoms stay in a single place, whereas all electrons can transfer across the nuclei, known as the “sea of electrons”. Due to this, metals are extremely conductive of electrical energy, since electrons and subsequently expenses are simply in a position to transfer round and be transferred all through it.

The intramolecular forces and interactions between molecules that create them are closely concerned with different subjects of chemistry. Which kind of intramolecular power a molecule has goes on to impact the way it interacts with different molecules round it, and thus the properties of any substance made up of it. Due to this, understanding intramolecular forces is vital to having a deeper understanding of what number of points of chemistry works.
[ad_2]