Utilizing earth ample supplies for lengthy period power storage: electro-chemical and thermo-chemical biking of bicarbonate/formate
[ad_1]
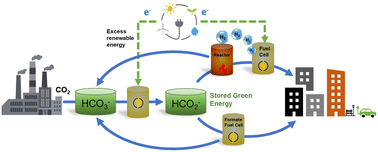
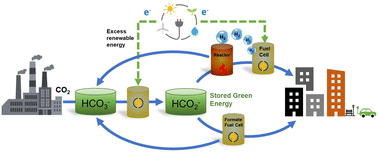
Utilizing hydrogen to retailer power in chemical bonds is a key part of the worldwide technique to reaching a sustainable future and ameliorating local weather change. The challenges related to dealing with molecular hydrogen will be solved through the use of liquid hydrogen carriers. On this perspective, we focus on the idea of bicarbonate–formate cycle, the place aqueous options of formate ions (HCO2−) are used as hydrogen and power carriers. Such options are composed by earth ample parts and, in distinction to widespread liquid natural carriers, are non-flammable, and readily convert to the oxide kinds (HCO3−) below response with water to launch hydrogen, or electrons, at average temperatures. We focus on thermodynamic facets of the bicarbonate–formate cycle and present the way it gives the chance of mixing electrochemical and thermochemical operations, in addition to of coupling CO2 seize with power/hydrogen storage. We emphasize the potential function of electrochemistry within the era of formate and within the launch of power within the type of electrical energy. At current, extra info on each the basic and techniques stage, is required to establish the possible situations for utilizing formate/bicarbonate salts for hydrogen/power storage. It’s doubtless, nevertheless, that a number of methods, together with hybrid electrochemical–thermochemical approaches, will go well with completely different functions. It’s also clear that extra integration between the disciplines of electrochemistry and heterogeneous catalysis is required to beat the challenges for advancing the HCO3−–HCO2− system as a possible inexperienced various for storing and transporting power.
[ad_2]