What Is A Mole In Chemistry?
[ad_1]
A mole is an SI unit used primarily in chemistry for measuring massive portions of very small entities like atoms, molecules, and ions. It’s the unit equal of a selected fastened amount of a substance, normally in grams. One mole is the same as Avogadro’s quantity, which has been experimentally decided to be 6.02214076 × 1023.
Calculating a mole could be very helpful when making ready exact quantities of gear from reactants as a result of it prevents materials waste and maximises effectivity. This, in flip, will help to make the chemical manufacturing course of more cost effective.
How is a mole outlined?
A mole is normally expressed in grams, which include roughly the variety of unit particles of Avogadro’s quantity, or 6.02214076 × 1023. For instance, a mole of carbon is 12 grams as a result of a carbon atom has an atomic weight of 12. Equally, a mole of water is equal to roughly 18 grams as a result of a water molecule has a molecular weight of 18 items.
It’s vital to notice the distinction between a mole and molar focus. Molar focus, which is usually abbreviated merely as M, is equal to the proportional mole mass in grams of a substance dissolved in a single litre of water or different equal solute-solvent proportions.
The fantastic thing about utilizing water as a solvent is that it could actually dissolve a variety of gear. Moreover, the quantity and mass of pure water have an nearly one-to-one relationship, with one mL or 1 cm3 of water equal to 1 gram beneath normal circumstances. As you possibly can think about, this makes the calculations for molar focus very handy!
How is a mole calculated?
A mole is at all times equal to Avogadro’s quantity when it comes to the variety of particles. Nonetheless, the mass equal in grams varies relying on the unit mass of the particles concerned. For example, it may very well be the mass equal of 1 mole of electrons, ions, atoms, or molecules.

The very first thing you could do to calculate the mole or molar mass of a substance is to find out the mass unit of its constituent entities per one particle unit.
Let’s use glucose, with its atoms because the constituent components, for instance. Every molecule of glucose consists of sure proportions of carbon, hydrogen, and oxygen.
The chemical formulation of glucose is C₆H₁₂O₆. Utilizing the periodic desk of components, we will decide the atomic mass of every ingredient of glucose. Then we occasions the mass of every ingredient by the subscript. The ultimate step is so as to add the person merchandise collectively to get the full mass of the molecule.
Right here’s the calculation:
| Component Composition | Variety of Atoms per Molecule | Atomic Mass (rounded off) | Complete Mass |
| Carbon | 6 | 12 | 72 |
| Hydrogen | 12 | 1 | 12 |
| Oxygen | 6 | 16 | 96 |
| TOTAL molar mass: | 180 |
As you possibly can see, a mole of glucose is roughly 180 mass items or 180 grams per mole. The precise worth is experimentally decided to be 180.156 g/mole, though for simplicity the quantity might be rounded off to its nearest complete quantity.
How are moles utilized in chemistry?
Moles are utilized in chemistry as a measure of the quantity of any given substance. The SI mole unit is especially helpful in calculating the exact amount of reactants which might be wanted to supply a selected quantity of product. It can be used for analytical functions to find out the chemical identification of an unknown pattern of a substance.
Typically, chemists use moles to calculate and put together a particular molar focus of a dissolved substance in an aqueous answer. Contemplate the next instance of a easy chemical response:
CaCO3 + 2HCl → CaCl2 + CO2 + H2O
On this case, you want two moles of hydrochloric acid (HCL) for each mole of calcium carbonate (CaCO3) to supply one mole of carbon dioxide (CO2). To organize, say, 0.25 M of carbon dioxide, the molar focus of hydrochloric acid would subsequently have to be 0.5 M.
In contrast to easy arithmetic additions, including two chemical substances collectively is about fastened and balanced proportions. The regulation of conservation of matter and power at all times applies in each chemical response. In lots of instances, the proportions are zero as a result of there are not any chemical reactions.
What are examples of a mole in chemistry?
The SI unit of mole relies on the metric system and Avogadro’s quantity. You should utilize empirically-determined mass items of particles like electrons, atoms, ions, and molecules to calculate mole and molar concentrations.
Examples of a mole in chemistry embody the mole values of every ingredient, the mole worth of water (18.0146 grams), of electrons, ions, and the mole worth of particular person molecules in a substance.
Though mole is a standardised unit of measurement, the idea might be higher understood utilizing non-standard items. Listed here are some examples.
1. Paper
A mole might be likened to a ream of paper. Identical to a mole represents a sure variety of atoms or molecules, a ream is often equal to 500 particular person sheets of paper.
2. Eggs
A regular carton of eggs can be in comparison with the mole unit of measurement. On this case, one carton composed of twelve or a dozen eggs might be thought-about as one unit of measurement.

3. Pencils
Pencils are generally offered in bins of 10 (though this may increasingly range relying on the type and model). So simply as one mole is equal to six.02214076 × 1023 particles, one field is the same as 10 items (pencils).
4. Water
Chemically, a mole of water is eighteen.01528 g/mol. Once more, it’s the equal of 6.02214076 × 1023 items or particular person molecules. Nonetheless, there are other ways of measuring items of water together with litres, millilitres, pints, quarts, and gallons.
What’s the formulation of moles?
Calculating the mole based mostly on Avogadro’s quantity is sort of easy. You merely have to divide the mass of a pattern of a substance by the calculated molar mass.
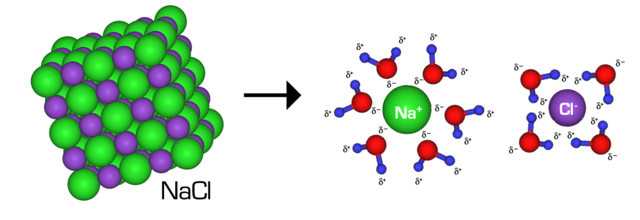
For all substances which have the identical molar mass because the pattern substance, the mole is precisely one. Nonetheless, if the mass of the pattern substance is completely different to the calculated molar mass, the mole worth is calculated proportionally. For example, a pattern of pure desk salt (sodium chloride) with a mass of 29 grams has a mole worth of 0.537 or equal to three.234 x 1023 molecules of salt.
Within the above instance, you merely have to divide 29 grams by the molar mass of sodium chloride, which is 58.44 g/mole. This provides you the mole worth. When you’ve calculated the quotient, you then multiply it by Avogadro’s quantity to find out the variety of atoms.
Abstract
In chemistry, a mole is an SI unit that’s used to measure massive quantities of very small entities like molecules and atoms. One mole is the same as Avogadro’s quantity, which is outlined as 6.02214076 × 1023. A mole is a really helpful idea as a result of it permits chemists to synthesise exact quantities of gear, minimise waste, and maximise effectivity.
[ad_2]