
A high-pressure flux technique to synthesize high-purity oxyhydrides
[ad_1]
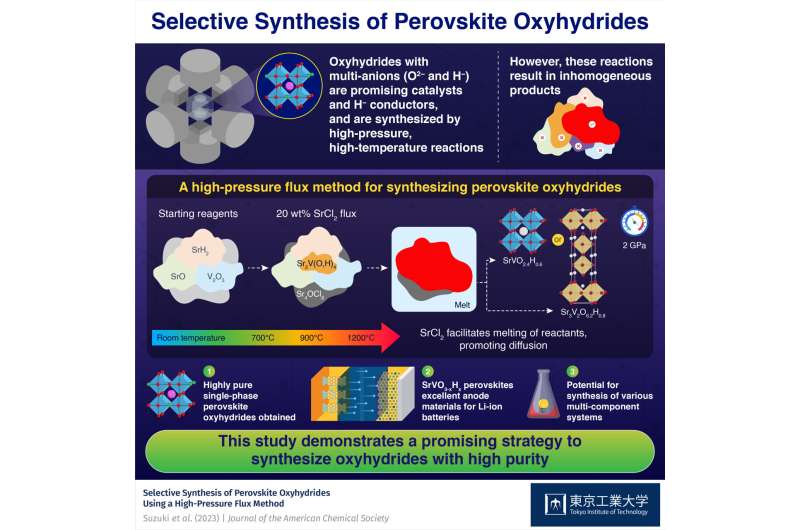
Including a flux in the course of the synthesis of oxyhydrides is a promising technique to get hold of a pure, homogenous product, reveal scientists from Tokyo Tech.
An SrCl2 flux promoted the melting of part of reactants and facilitated their diffusion of reactants, which proved to be the important thing to producing extremely pure SrVO2.4H0.6 or Sr3V2O6.2H0.8 perovskite oxyhydrides in high-pressure and high-temperature reactions. These compounds have potential as catalysts and as electrode supplies for lithium-ion batteries.
Perovskite oxyhydrides containing oxide (O2–) and hydride (H–) anions are promising compounds with purposes in catalytic techniques and batteries. Sadly, synthesizing oxyhydrides is often fairly difficult, primarily because of the extremely reactive nature of H– anions.
It was identified that high-pressure and high-temperature reactions are efficient to synthesize oxyhydrides. For instance, Sr2VO4–xHx perovskite might be synthesized instantly from oxide and hydride precursors in high-pressure and high-temperature reactions.
A key benefit of those reactions is that the H– content material within the closing product might be tuned by adjusting the composition and ratio of the precursors. This primarily implies that the digital and magnetic properties of the product are additionally customizable.
Not like Sr2VO4–xHx, synthesizing SrVO3–xHx has confirmed far more tough, because the needed high-pressure and high-temperature reactions result in the formation of a number of impurities and inhomogeneous merchandise, primarily on account of inadequate diffusion of the stable parts.
In a current research revealed in Journal of American Chemical Society, a analysis group led by Affiliate Professor Takafumi Yamamoto from the Institute of Progressive Analysis at Tokyo Institute of Know-how (Tokyo Tech) discovered an answer to this downside. They developed a novel strategy to synthesize extremely pure SrVO2.4H0.6 and Sr3V2O6.2H0.8, two new perovskite oxyhydrides. This research was performed as a part of a collaborative analysis challenge with the Nationwide Institutes for Quantum Science and Know-how, Japan.
The researchers began with SrO, SrH2, and V2O3, and added SrCl2 to those reactants. They noticed the variations within the composition of samples ready beneath completely different situations utilizing a method referred to as in-situ synchrotron X-ray diffraction, shedding gentle on the function of SrCl2 within the response. It acted as a flux at a excessive temperature of 1200 ℃ and a excessive strain of two GPa, facilitating the melting and dissolution of part of reactants, thus selling diffusion.
Consequently, the researchers managed to suppress the event of inhomogeneous merchandise that usually seem on account of inadequate diffusion, acquiring extremely pure SrVO2.4H0.6 or Sr3V2O6.2H0.8 perovskite oxyhydrides.
Moreover, the group analyzed the electrochemical properties of the ready perovskite oxyhydrides as an electrode materials. “With low working potential, glorious reversibility, and high-rate traits, SrVO3–xHx may very well be appropriate as a destructive electrode for lithium-ion batteries, a primary for oxyhydrides,” says Dr. Yamamoto.
Total, utilizing a flux to spice up the specified response pathways in high-pressure and high-temperature reactions may very well be a strong technique to unlock a plethora of latest compounds past perovskite oxyhydrides. Dr. Yamamoto says, “The proposed synthesis strategy would even be efficient within the synthesis of assorted varieties of multi-component techniques.”
Extra data:
Selective Synthesis of Perovskite Oxyhydrides Utilizing a Excessive-Strain Flux Methodology, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c02240
Offered by
Tokyo Institute of Know-how
Quotation:
A high-pressure flux technique to synthesize high-purity oxyhydrides (2023, July 25)
retrieved 25 July 2023
from https://phys.org/information/2023-07-high-pressure-flux-method-high-purity-oxyhydrides.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.
[ad_2]






