Affect of Intermolecular Forces | ChemTalk .
[ad_1]
Core Ideas
On this article, you’ll study in regards to the results of intermolecular forces (IMFs). You’ll perceive how intermolecular forces affect the bodily properties of matter, resembling melting level, viscosity, and floor stress.
Subjects Coated in Different Articles
What are Intermolecular Forces?
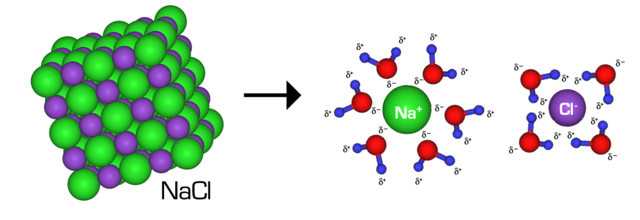
Intermolecular forces (IMFs), as its title would counsel, are forces between molecules. They’re electrostatic interactions between charged molecules. Although IMFs embody each engaging and repulsive interactions, the time period is often solely used for engaging interactions between molecules (having robust IMFs between two molecules means the pressure of attraction between the 2 is robust).
There are several types of intermolecular forces: London dispersion forces, dipole-dipole forces, ion-dipole forces, and hydrogen bonding. Intermolecular forces have various strengths, and the power of the intermolecular forces inside a substance influences the substance’s bodily properties, together with boiling/melting factors, viscosity, floor stress, vapor strain, and many others.
How Intermolecular Forces Affect Bodily Properties
Boiling/Melting factors
The boiling level is the temperature at which a liquid can flip right into a gasoline, and the melting level is the temperature at which a stable can flip right into a liquid.
Having robust intermolecular forces signifies that the attraction between the molecules is robust. Subsequently, if a substance has stronger IMFs, it’s more durable to tug its molecules away from one another, ensuing within the substance having a better boiling level and melting level.
For instance, propane (C3H8), has a boiling level of -42°C whereas methane (CH4), has a boiling level of -161.6°C. Propane has such a better boiling level than methane as a result of it has stronger London dispersion forces (bigger molecule) and stronger IMFs general than methane.
Viscosity
One other impact of intermolecular forces is viscosity. Viscosity is the measure of a fluid’s resistance to stream. A excessive viscosity leads to a thicker and stickier fluid. Viscosity is a results of intermolecular forces. Stronger intermolecular forces trigger a fluid to have a stronger attraction to itself, giving it a excessive viscosity.
Glycerol, for instance, has a lot stronger intermolecular forces than water as a result of it incorporates 3 -OH hydroxyl teams able to hydrogen bonding, whereas water solely has one. That is why water flows very properly whereas glycerol doesn’t.

Vapor Stress and Volatility
The power of the intermolecular forces of a liquid impacts the liquid’s vapor strain. Vapor strain is the strain exerted by vapor on the floor of a liquid. A rise in volatility, which is the tendency of a substance to evaporate, leads to a rise in vapor strain. Stronger intermolecular forces give a liquid a stronger attraction to itself. This makes it more durable for the liquid to evaporate, leading to decrease volatility and decrease vapor strain.
Floor Pressure
One of many results of intermolecular forces is floor stress. Floor stress is the elastic property of the floor of a liquid, resembling how water drops type a spherical form. This property is attributable to intermolecular forces, which hold the molecules collectively and trigger the floor of the liquid to withstand exterior forces. The stronger the intermolecular forces, the better the floor stress.

Solubility
One other one of many results of intermolecular forces is solubility. The stronger the intermolecular forces between the solute and solvent, the better the solubility. The phrase “like dissolves like” helps predict the solubility of a solute in a solvent. Polar molecules dissolve polar molecules via dipole-dipole forces, and nonpolar molecules dissolve nonpolar molecules via London dispersion forces. Polar molecules and nonpolar molecules don’t dissolve properly with one another.
Affect of Intermolecular Forces Follow Issues
Order the next compounds from lowest boiling level to highest:
- Helium gasoline He
- Isobutyl alcohol C4H10O
- Acetone (CH3)2CO
- Water H2O
Affect of Intermolecular Forces Options
Resolution: Helium gasoline, Acetone, Water, Isobutyl alcohol
Clarification: Since having stronger intermolecular forces will increase boiling level, this downside is to rank the compounds from weakest to strongest intermolecular forces. Helium Gasoline is the smallest molecule right here and is barely able to London dispersion forces, so it has the bottom boiling level. Acetone is able to dipole-dipole forces however not hydrogen bonding, which water and Isobutyl alcohol are able to, so acetone has the second lowest boiling level. Water and Isobutyl alcohol each have one -OH hydroxyl group, however Isobutyl alcohol is bigger and has stronger London dispersion forces, so Isobutyl alcohol has the very best boiling level.
[ad_2]