Arenes | ChemTalk
[ad_1]
Core ideas
On this matter, you’ll find out about arenes, their construction, guidelines of nomenclature, and the orientation of electrophiles.
Matters Lined in Different Articles.
What are Arenes?
Arenes are cyclic fragrant hydrocarbons, containing alternating single and double-bond between the carbons. The system of two or extra pi bonds separated by a single bond is named the conjugate system. The presence of the conjugate system is a key function that separates arenes from different cyclic constructions.
Benzene (C6H6), is the best instance of arene.
In benzene, the conjugate pi bond resonates freely within the cyclic ring and provides rise to the resonance constructions of benzene. The delocalized pi electrons shifting from one carbon to a different present stability to the benzene ring.
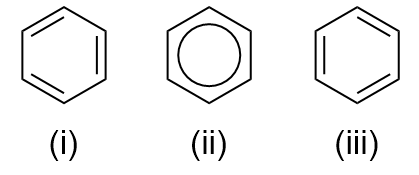
Kekulé Construction of Benzene
Friedrich August Kekulé, a German scientist supplied foundation concerning the construction of benzene. On this construction, alternating single and double bonds between adjoining carbon kind a six-membered hexagonal ring with a hydrogen atom bonded to every carbon. Kekulé goal the thought of two constructions the place there’s an oscillation between the 2 varieties.

The oscillation of double bonds in benzene may be defined with the assistance of resonance. In keeping with resonance principle, the mix of a number of potential constructions represents the molecule. In benzene, the delocalization of electrons provides rise to 2 interconvertible constructions often called resonating constructions. From X-ray diffraction research, the C-C bond size in benzene was discovered to be equal to 1.39 Angstroms which is the intermediate bond size between carbon-carbon single (1.54) and double (1.34) bonds. Thus, although the 2 resonating constructions are equal to at least one one other, the true construction of benzene is given by the resonance hybrid.
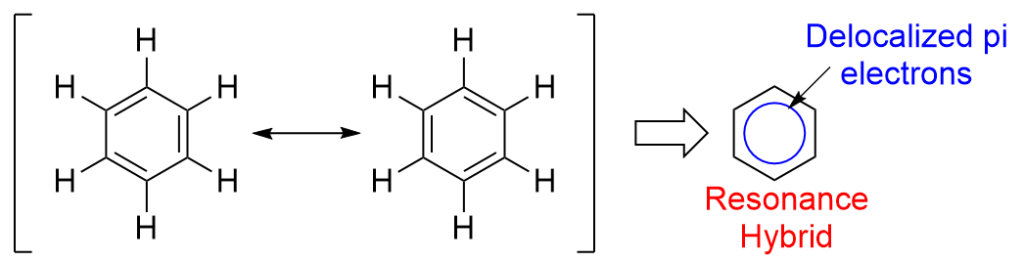
Resonance of benzene
There are two principle that explains the prevalence of resonance in benzene.
Valence bond principle: Every carbon atom in benzene has three sp2 hybrid orbitals and one unhybridized 2pz orbital. Out of the three sp2 hybrid orbitals, two take part in C-C σ bond, whereas the remaining one varieties C-H σ bond. Thus, there are a complete of 12 σ bonds. The remaining unhybridized 2pz orbital varieties π bond with the adjoining carbon by way of lateral overlap. There may be an equal risk of π bond formation by all 6 carbon atoms within the ring which explains the formation of two resonating constructions proposed by Kekulé.
Molecular orbital principle: Much like valence bond principle, 2pz orbitals are chargeable for the formation of π bond. Right here, the six unhybridized 2pz orbital overlap to generate 6 molecular orbitals (3 bonding and three anti-bonding). The like part orbitals (bonding to bonding, and anti-bonding to anti-bonding ) mix to kind a electron cloud above and beneath the ring.
Prediction of aromaticity in Arenes.
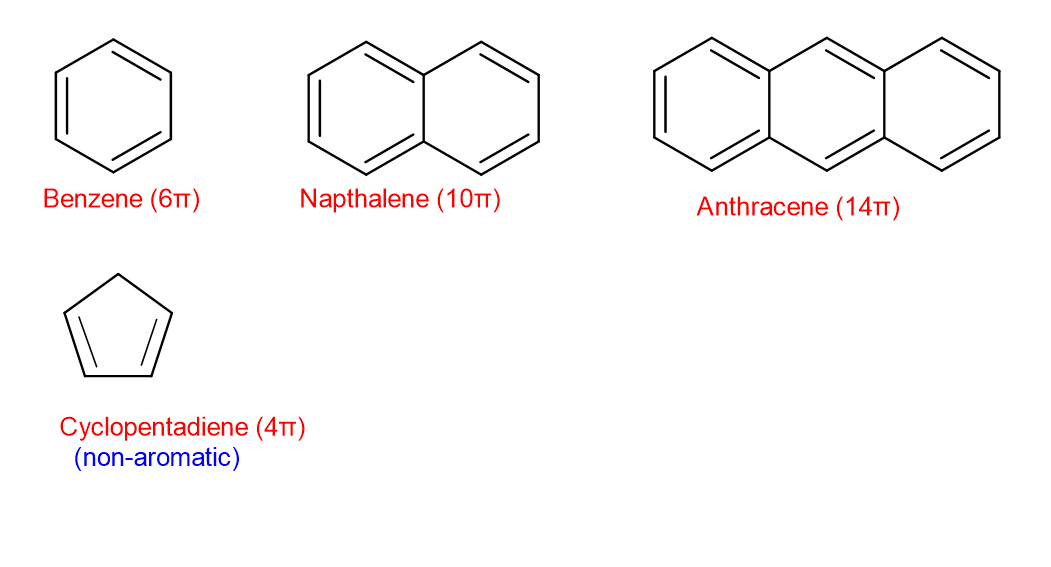
Huckel’s rule states {that a} conjugated ring system containing (4n + 2) π electrons will present fragrant character. (n is a constructive integer) .
When n=1, the conjugated system has 6π electrons. Equally, when n=2, the system has 10π electrons and when n=3, the conjugated system has 14π electrons.
So, the system of 6π , 10π ,14π electrons and so forth are fragrant.
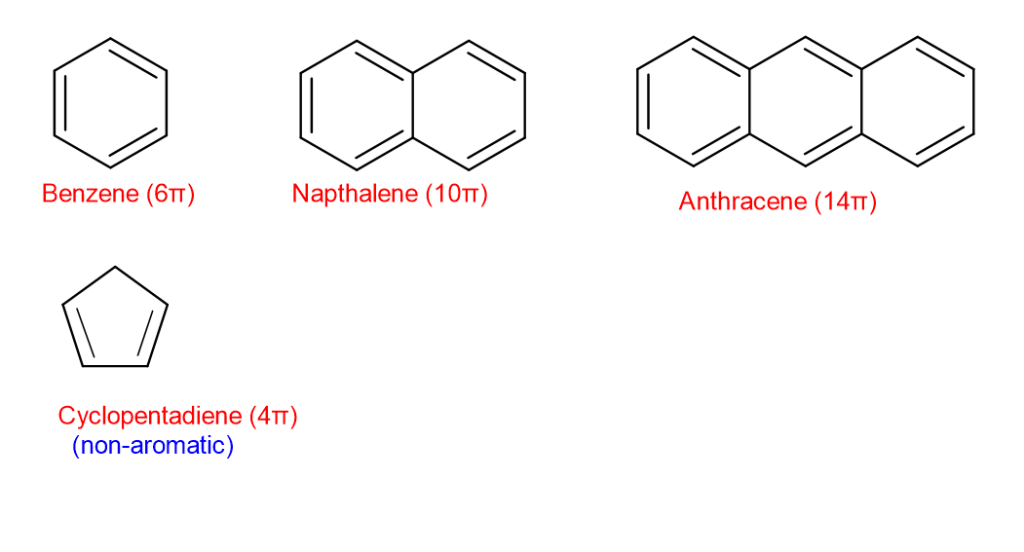
Nomenclature
Whereas naming substituted benzene the substituent turns into a prefix to the phrase root benzene.
Chlorine – Chloro+benzene= Chlorobenzene
Nitro group- Nitro+benzene= Nitrobenzene

For disubstituted benzene. The naming is completed as, (place of substituent)+ di+ (substituent) + benzene.
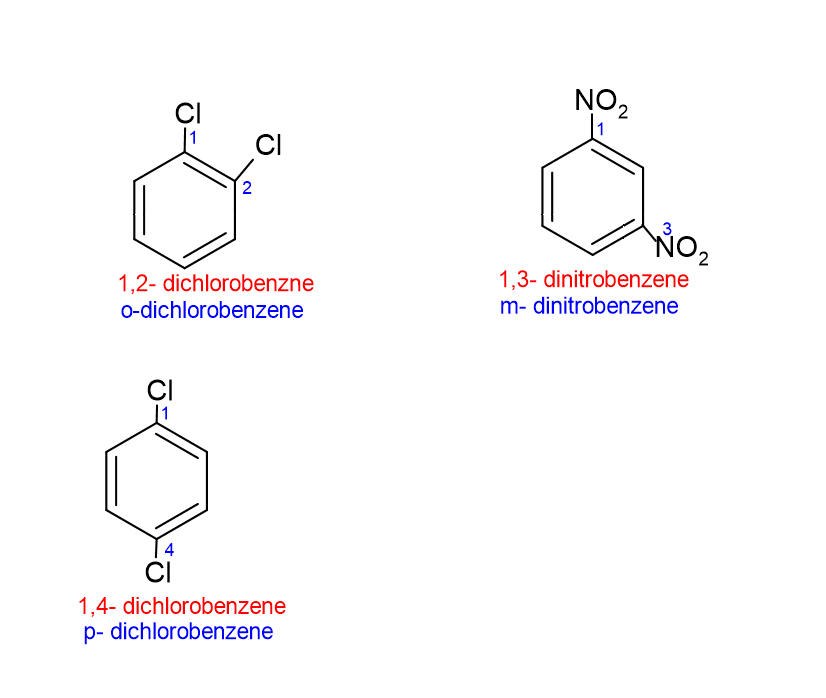
As an alternative of utilizing numbers for disubstituted benzene, ortho (o) ,meta (m) and para (p) can mark the positions.
- 1,2 positions – ortho (o) place
- 1,3 positions – meta (m) place
- 1,4 positions – para (p) place

Equally for trisubstituted benzene.
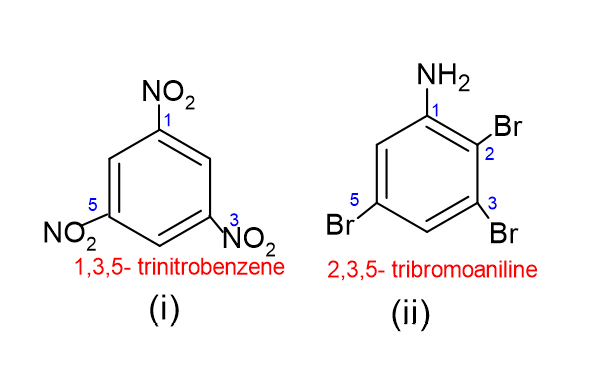
For (ii) construction, aniline is used as an alternative of benzene because of the presence of NH2 group.
Phenyl group
Phenyl group is a cyclic group of atoms with the formulation C6H5 and represented by the image Ph. The alternative of a hydrogen atom by a substituent in benzene varieties a phenyl group (R-C6H5).
Benzyl group
Benzene with a methylene (CH2) group is named benzyl. It’s represented by a basic formulation. R−CH2−C6H5.
Throughout nomenclature, each phenyl and benzyl are used as prefix.
Fragrant substitution
The group current within the benzene ring decides the place of the coming into teams. Chemists name this phenomenon the directive impact. The phrases administrators or directing teams describe the teams already current.
1. Ortho- and para-directors: They direct the electrophile towards ortho and para positions. -OH,-NH2, -Cl, -CH3 belong on this class. Ortho- and para-directors are electron-donating and ring activating ring-activating teams. (Aside from halogen which is a hoop deactivator) .
2. Meta-directors: They’re electronegative atoms (electron-withdrawing group) that direct the electrophile in the direction of meta place. They’re meta-directing and ring-deactivating group. For instance, -COOH, -CHO , – CN
Arenes Follow Issues
Downside 1
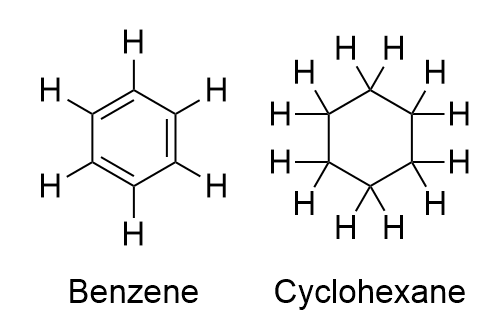
Why is cyclohexane non-aromatic:
Downside 2
What’s the distinction between phenyl and phenol?
Downside 3
Why are sure teams ortho and para administrators whereas others are meta administrators?
Arenes Follow Issues Answer
1: Cyclohexane include solely C-C single bond, on account of which there aren’t any pi electrons current. The absence of pi electrons makes it non-aromatic.
2: The alternative of a hydrogen atom by a substituent in benzene varieties a phenyl group (R-C6H5). Whereas, phenol is the alternative of hydrogen by a hydroxyl group (-OH).
3: The o/p administrators are an electron donating group. They donate electrons to the ring which causes the electron density to be excessive on the ortho and para positions . That is why electrophiles desire to assault at ortho and para positions.

Simillary, the meta administrators are electron withdrawing teams. They withdraw electrons and deactivate the ring. On this case, the electron density is most at meta place (no constructive cost at meta place) , thus the electrophile assault the meta place.

[ad_2]