Controlling the toxicity of biomass-derived difunctional molecules as potential pharmaceutical components for particular exercise towards microorganisms and mammalian cells
[ad_1]
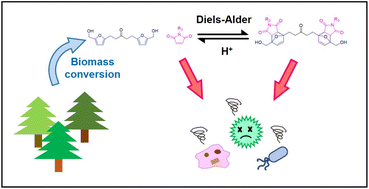
A biomass-derived difuran compound, denoted as HAH (HMF–acetone–HMF), synthesized by aldol-condensation of 5-hydroxyfurfural (HMF) and acetone, may be partially hydrogenated to offer an electron-rich difuran compound (PHAH) for Diels–Alder reactions with maleimide derivatives. The nitrogen (N) web site within the maleimide may be substituted by imidation with amine-containing compounds to manage the hydrophobicity of the maleimide moiety in adducts of furans and maleimide by Diels–Alder response, denoted as norcantharimides (Diels–Alder adducts). The structural results on the toxicity of varied biomass-derived small molecules synthesized on this method to control organic processes, outlined as low molecular weight (≤1000 g mol−1) natural compounds, have been investigated towards various microbial and mammalian cell sorts. The organic toxicity elevated when hydrophobic N-substitutions and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds have been launched into the molecular construction. Among the many synthesized norcantharamide derivatives, some compounds demonstrated pH-dependent toxicities towards particular cell sorts. Response kinetics analyses of the norcantharimides in organic circumstances recommend that this pH-dependent toxicity of norcantharimides might come up from retro Diels–Alder reactions within the presence of a Brønsted acid that catalyzes the discharge of an N-substituted maleimide, which has greater toxicity towards fungal cells than the toxicity of the Diels–Alder adduct. These artificial approaches can be utilized to design biologically-active small molecules that exhibit selective toxicity towards varied cell sorts (e.g., fungal, most cancers cells) and supply a sustainable platform for manufacturing of prodrugs that would actively or passively have an effect on the viability of infectious cells.
C bonds have been launched into the molecular construction. Among the many synthesized norcantharamide derivatives, some compounds demonstrated pH-dependent toxicities towards particular cell sorts. Response kinetics analyses of the norcantharimides in organic circumstances recommend that this pH-dependent toxicity of norcantharimides might come up from retro Diels–Alder reactions within the presence of a Brønsted acid that catalyzes the discharge of an N-substituted maleimide, which has greater toxicity towards fungal cells than the toxicity of the Diels–Alder adduct. These artificial approaches can be utilized to design biologically-active small molecules that exhibit selective toxicity towards varied cell sorts (e.g., fungal, most cancers cells) and supply a sustainable platform for manufacturing of prodrugs that would actively or passively have an effect on the viability of infectious cells.

[ad_2]